Update to the Joint Statement on COVID-19 From Lung Cancer Advocacy Groups
November 23rd, 2020
From the Go2Foundation for Lung Cancer website:
We are at a critical moment in the ongoing COVID-19 pandemic. New cases are rapidly escalating throughout the country, and we are positioned to see explosive growth as people travel and gather to celebrate the Thanksgiving holiday with loved ones. While our understanding of how to treat COVID-19 has grown significantly since the disease first burst onto the scene, deaths continue to mount, with the US now seeing the most daily deaths since May.
The realities of the current situation are compounded by our collective national fatigue and desire to return to some sense of normalcy. When we look at website hits for these joint statements over time, we see a lot of activity in the spring when COVID-19 was “new,” but those numbers have dropped off substantially through the summer and fall. This stands in stark contrast to the growth of cases through subsequent waves of infection.
The take home message is that we must not let our guard down! Please continue to wear a mask, watch your distance and wash your hands. Our collective actions over the next few weeks CAN make a difference in helping curb the recent surge. We also recognize the importance of balance, particularly for patients with cancer who fear they may not have another Thanksgiving or Christmas. For practical guidance on how to navigate your holidays safely, please refer to this helpful discussion.
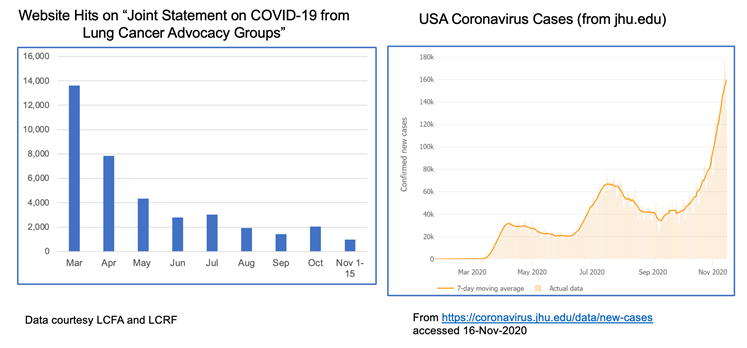
Despite the current situation, there is reason for hope. We can now see the light at the end of the tunnel with the recent announcements that both Moderna and Pfizer/BioNTech have developed highly effective COVID-19 vaccines, with others in the pipeline. You can find a comprehensive overview of how vaccine trials work and current vaccine efforts underway here.
Additionally, monoclonal antibody therapies continue to make progress. Eli Lilly recently received Emergency Use Authorization from the FDA for its antibody therapy in recently diagnosed, high-risk patients. Regeneron also received a lot of press when its antibody therapy was used to treat President Trump.
VACCINE FAQS
The development of a new class of mRNA-based vaccines has raised many questions, particularly among the lung cancer community. We have been collecting these questions and will do our best to address them here.
1) How do mRNA vaccines work?
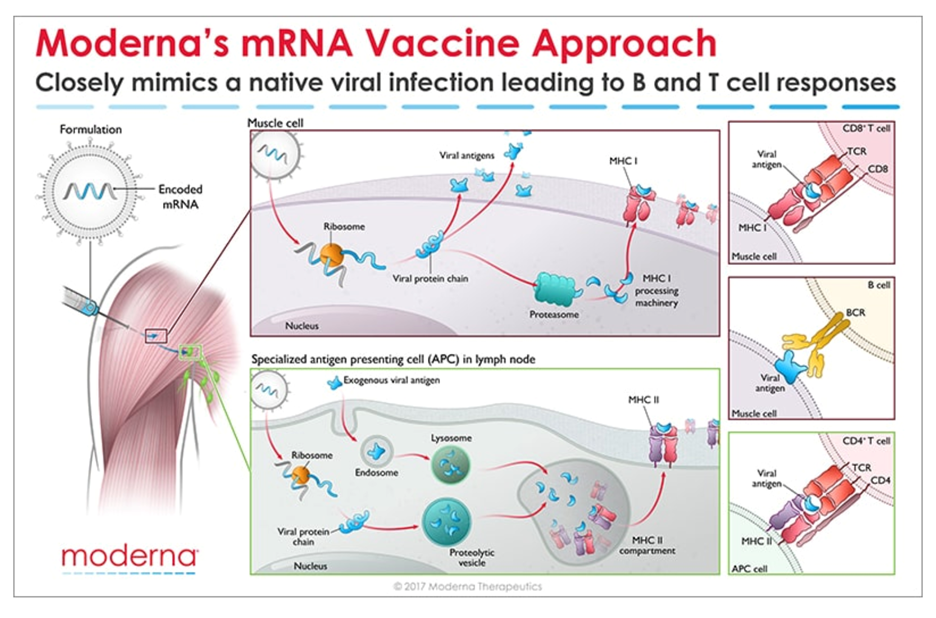
Messenger RNA (mRNA) is the recipe for making a protein. The mRNA gets injected into the body and is taken up by cells that “read the recipe” for making the SARS-CoV-2 spike protein. This is the protein normally expressed as a “crown” on the virus particle and is the part of the virus that binds to the receptor found on cells in the lungs and in other tissues throughout the body. Once these cells take up the mRNA and make the spike protein, they can display pieces of spike on their cell surface to signal the immune cells to become activated. B cells are a type of immune cells that make antibodies that can block virus binding. CD4 T cells support B cells to make antibodies while CD8 T cells can kill virus-infected cells. This is illustrated in the figure below for Moderna’s vaccine (though Pfizer/BioNTech’s vaccine works in the same manner).
2) How do we know these vaccines are safe?
All new drugs and vaccines go through extensive testing as part of the clinical trials process. (summarized in the NYTimes link above). Both the Moderna and Pfizer/BioNTech vaccines are currently in Phase 3 clinical trials, reporting nearly 95% efficacy and no significant safety issues. It is important to note that these trials have been conducted in thousands of patients. However, no significant safety issues does not mean the vaccines don’t come with some unpleasant side effects which are short-lived. Those effects should not be a reason to avoid the vaccine. Educating healthcare providers on the mRNA technology and ensuring them that the vaccines are safe will be key to a successful rollout.
3) When will the vaccines be available? Will patients with lung cancer be prioritized?
Based on the safety and efficacy profiles of both vaccines, it is expected that people will start receiving them before the end of the year, perhaps as soon as December 12 in the US. Many national experts are developing guidance for vaccine distribution, with the National Academies issuing a framework that would see healthcare workers, frontline workers and those in high-risk categories being eligible to be vaccinated first. Given that several studies have now reported high mortality rates in patients with lung cancer who contract COVID-19 , it is widely expected that lung cancer patients would be among those first eligible to receive the vaccine in the early stages of rollout.
4) Should I take the first vaccine available or wait for a later generation one?
As stated earlier, both the Moderna and Pfizer/BioNTech vaccines are highly effective with a strong safety profile. There have been fears among many that the rush to produce a vaccine would result in compromised safety or efficacy but adherence to standards established by the FDA and other agencies assures us that these vaccines are safe.
It is important to note that before mRNA vaccines were developed in the fight against COVID-19, they were being developed to help combat cancer. Both Moderna and BioNTech (the company that partnered with Pfizer on its COVID-19 vaccine) have been developing mRNA vaccine technology for some time in the hopes of using this approach to treat various forms of cancer as well as other infectious diseases.
Given the unique threat that COVID-19 presents to the lung cancer community, we strongly encourage you to have a discussion with your doctor about getting the vaccine as soon as it is available to you. As for choosing between these two specific vaccines, the technology is essentially identical. Both require two shots over the course of a few weeks. The differences come down to logistical challenges of ensuring facilities have proper freezers for maintaining the vaccines at the appropriate subzero temperatures.
Other vaccine candidates are in development that use different technology platforms. It remains possible that some future vaccines may require only a single dose (such as Janssen’s vaccine) or be administered differently (intranasal vs injection).
Until those vaccines gain approval, the current decision will be based on availability of the two mRNA-based vaccines.
It is worth noting that a multi-institutional, NCI-funded grant has been awarded to study antibody responses to SARS-CoV-2 infection in lung cancer patients as compared to healthy people. This effort will try to answer why lung cancer patients seem to have worse outcomes from COVID-19 and will study responses in patients receiving a vaccine compared to those who do not.
UNANSWERED QUESTIONS
Several questions remain about the new mRNA vaccines:
- Can these vaccines completely prevent infection, or will they just prevent symptoms from developing?
- Can people who receive the vaccine still transmit the virus to others?
- How long will any resulting immunity last? Previous results from these types of vaccines in other settings suggest that protection may wane after a year.
More data is needed before we can answer these questions.
FINAL TAKEAWAY
There is no escaping the seriousness of our current national crisis – COVID-19 cases are increasing everywhere and so we must do what we can to protect ourselves and our loved ones a little while longer.
However, hope is on the horizon. We can face 2021 knowing that, through the power of science, this pandemic will eventually come to an end.
HAPPY HOLIDAYS AND PLEASE STAY SAFE!
Resources and websites
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute website for COVID-19 and emergency preparedness COVID-19: What People with Cancer Should Know
- Updates from the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- COVID-19 in patients with cancer: managing a pandemic within a pandemic
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers.
GO2 Foundation for Lung Cancer (Amy Moore, PhD – amoore@go2foundation.org)
LUNGevity Foundation (Upal Basu Roy, PhD, MPH – ubasuroy@lungevity.org)
Lung Cancer Foundation of America (Kim Norris – KNorris@lcfamerica.org)
Lung Cancer Research Foundation (Cristina Chin, LMSW, MPH – cchin@lcrf.org)
LungCAN (Kimberly Lester – kimberly@lungcan.org)


